1中國科學院上海藥物(wù)研究所
2中科院
3賽諾菲公司,美(měi)國DMPK部門
藥物(wù)相互作用(yòng)
結果:
人(rén)OATP1B1/1B3攝取轉運體(對(duì)應大(dà)鼠OATP1B2)将甘草(cǎo)酸從血液攝取進入肝髒,人(rén)外排轉運體MRP2、BCRP、BSEP、MDR-1(對(duì)應大(dà)鼠MRP2/BCRP/BSEP)介導藥物(wù)外排進入膽汁中。利福平(OATP1B抑制劑)将抑制大(dà)鼠肝髒攝取型轉運體,導緻甘草(cǎo)酸的(de)系統暴露量明(míng)顯增加;此外,甘草(cǎo)酸與血漿蛋白廣泛結合,其腎小球濾過率較低。最終導緻甘草(cǎo)酸體内暴露量較高(gāo)。PBPK模型的(de)定量分(fēn)析表明(míng)當甘草(cǎo)酸與轉運體抑制劑聯合給藥時(shí),甘草(cǎo)酸藥動學過程中發揮關鍵作用(yòng)的(de)OATP1B1/1B3将受到抑制,并引起潛在的(de)藥物(wù)相互作用(yòng)DDI風險。
結論:
轉運體介導甘草(cǎo)酸的(de)肝髒攝取與膽汁排洩,影(yǐng)響其消除與藥代動力學,定量分(fēn)析OATP1B1/1B3轉運體介導的(de)甘草(cǎo)酸潛在DDI風險,可(kě)以增強甘草(cǎo)酸與其他(tā)藥物(wù)合用(yòng)治療肝髒疾病的(de)成功率。
KEY RESULTS
Hepatobiliary excretion of glycyrrhizin involved human OATP1B1/1B3-(orOatp1b2inrats)mediated hepatic uptake from blood and human multidrug resistance-associated protein (MRP)2/breast cancer resistance protein (BCRP)/bile salt export pump (BSEP)/multidrug resistance protein(MDR)1-(orMrp2/Bcrp/Bsepin rats)mediatedhepaticefflux into bile. Impairment of hepatic uptakein rats by rifampin resulted insignificantly increased systemic exposure to glycyrrhizin, which had slow glomerular-filtration-based renal excretion due to extensive protein-binding in plasma. Quantitative analysis using the PBPK model demonstrated the critical roles of OATP1B1/1B3 in pharmacokinetics ofglycyrrhizin,which hadhigh likelihoodto be avictimof drug-drug interactions when coadministered with potent dual inhibitors of these transporters.
CONCLUSION AND IMPLICATIONS
Transporter-mediated hepatobiliary excretion governs glycyrrhizin’selimination and pharmacokinetics. Understanding glycyrrhizin’s potential drug-drug interactions on OATP1B1/1B3 is expected to enhance success of glycyrrhizin-including combination drug therapies of liver diseases.
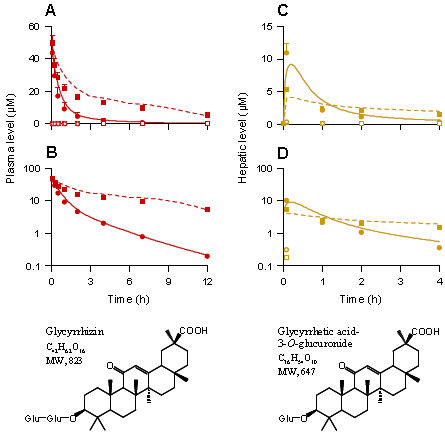
圓點代表不用(yòng)利福平處理(lǐ)空白對(duì)照(zhào)組大(dà)鼠,正方形點代表用(yòng)利福平處理(lǐ)的(de)大(dà)鼠,A和(hé)B圖代表大(dà)鼠靜脈注射2.6mg/kg 皂苷,血漿中甘草(cǎo)甜素和(hé)甘草(cǎo)酸-3-O-糖醛酸的(de)濃度-時(shí)間曲線及對(duì)數圖
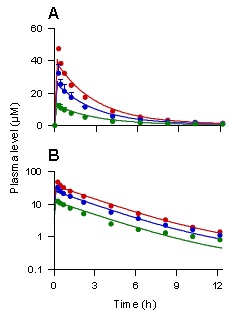
A和(hé)B圖分(fēn)别代表人(rén)靜脈滴注甘草(cǎo)甜素40mg(綠(lǜ)色線)、80mg(藍色線)、120mg(紅色線)後,血漿中甘草(cǎo)甜素的(de)濃度時(shí)間曲線圖及對(duì)數圖
下(xià)載該篇文章(zhāng)的(de)英文原文獻PDF文件: static/file/file (下(xià)載792)
 協同生物(wù)
協同生物(wù)







您好!請登錄